Key Moments:
- Lyra Therapeutics shares surged around 600% after the company reported that Phase 3 of its LYR-210 trial had been successful.
- ENLIGHTEN 2 trial met its primary goal, showing significant symptom improvement at 24 weeks.
- Management plans to pursue a New Drug Application for LYR-210 in patients without nasal polyps.
Breakthrough Results in ENLIGHTEN 2 Trial
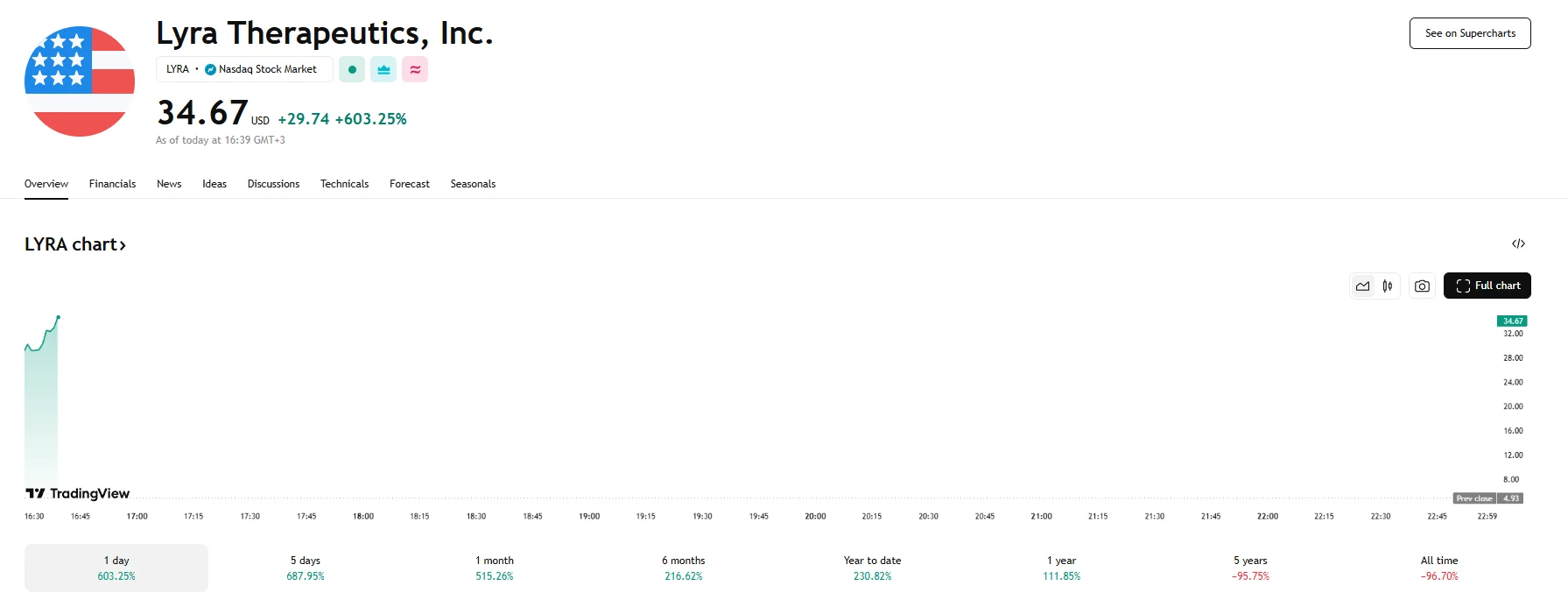
Lyra Therapeutics disclosed on Monday that its Phase 3 ENLIGHTEN 2 clinical trial had ended with favorable outcomes. The news bolstered confidence in the stock, with the share price surging 603.25% to $34.67 after US markets opened.
The study evaluated the company’s LYR-210 implant, which aims to treat chronic rhinosinusitis (CRS), and achieved its main efficacy objective. The data demonstrated a statistically significant improvement in the three cardinal symptoms of CRS, namely nasal obstruction, nasal discharge, and facial pain or pressure, at the 24-week mark with a probability value of 0.0078.
Additional Signs of Efficacy and Safety
Beyond its primary objective, ENLIGHTEN 2 achieved success in crucial secondary measures, including improved cardinal symptoms (p=0.0209) and a higher SNOT-22 score (p=0.0101) after 24 weeks. The treatment showed consistent improvements from week four through trial completion. LYR-210’s safety profile was favorable, aligning with the sham control.
Notably, the improvements were noticeable as early as four weeks into treatment and remained consistent until the end of the trial. In terms of safety, LYR-210 performed on par with the sham control, indicating a favorable safety profile.
Data from Lyra Therapeutics’ ENLIGHTEN 1 and 2 trials, combining 64 CRS patients with nasal polyps, showed generally positive efficacy trends over 24 weeks. Not all results were statistically significant, however.
Regulatory Strategy and Forward Plans
Lyra Therapeutics executives believe LYR-210 offers a promising six-month, single-dose solution for CRS patients resistant to existing treatments. The company intends to discuss a New Drug Application with the FDA for patients without nasal polyps, concurrently evaluating development strategies for those with polyps.
President and CEO of Lyra Therapeutics Maria Palasis remarked that the findings had demonstrated a capability to positively affect both non-polyp and polyp patients, simultaneously providing further validation for their platform in ear, nose, and throat (ENT) applications.